We provide the following services:
- Case (ICSR) Reporting: Processing, QC and Submission of
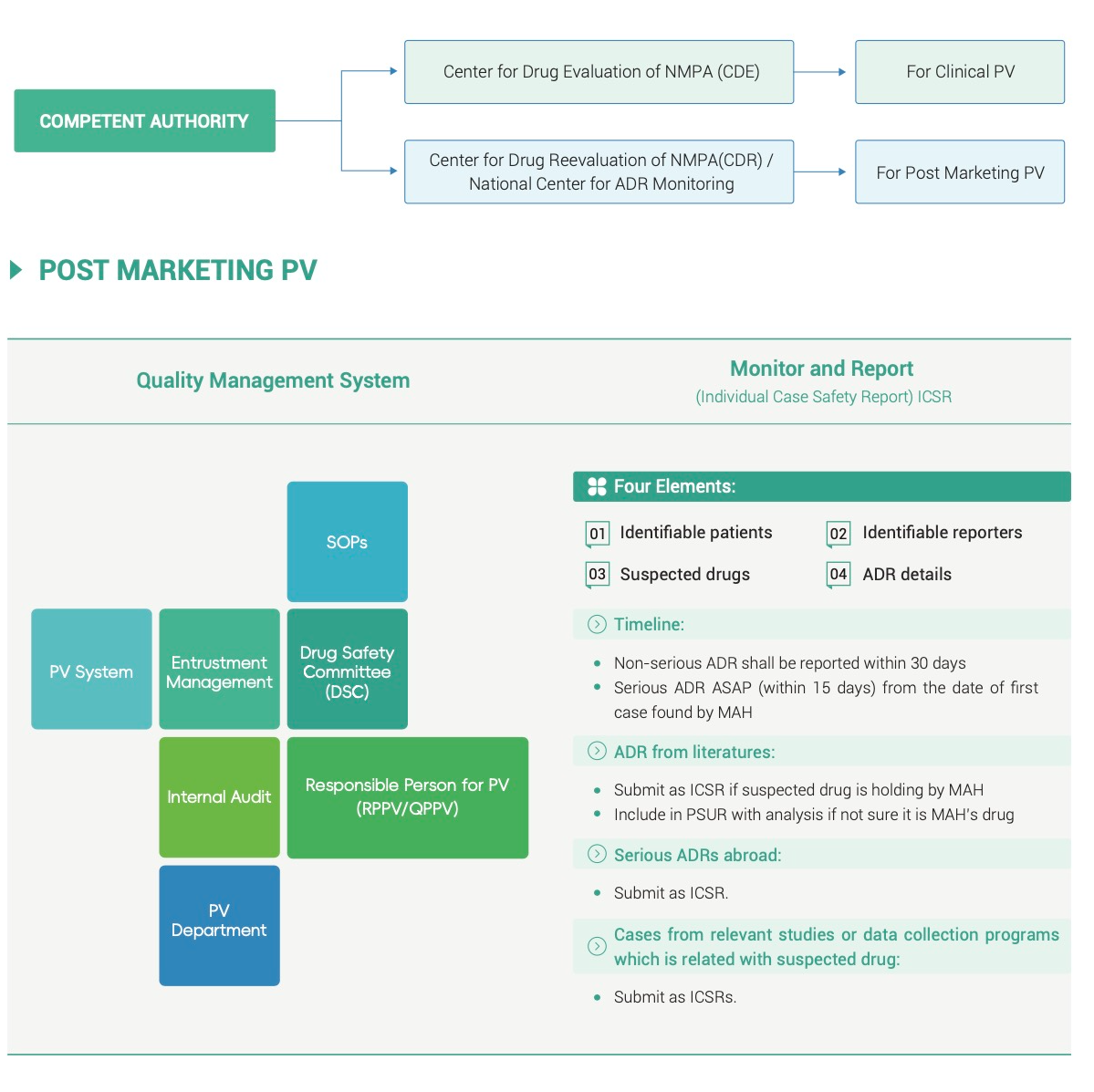
- Post-marketing Individual Case Safety Reports
- Literature Screening
- Signal Monitor and Risk Assessment
- China RPPV/QPPV Support (Responsible Person for Pharmacovigilance)
- Periodic Safety Update Report (PSUR)
- China Risk Management Plan (RMP)/Pharmacovigilance Plan
- PV Annual Report
- PV System Master Files (PSMF)
- Competent Authority Audit Support
- Team Training, Business Partners Overview
- SUSAR and DSUR (for Clinical PV)


 2006-2025 上海博華國際展覽有限公司版權(quán)所有(保留一切權(quán)利)
滬ICP備05034851號(hào)-57
2006-2025 上海博華國際展覽有限公司版權(quán)所有(保留一切權(quán)利)
滬ICP備05034851號(hào)-57