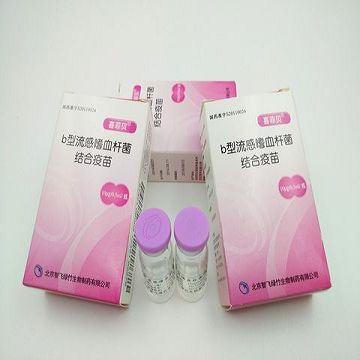
Haemophilus Influenzae Type b Conjugate Vaccine (Hib) is the product developed independently by Zhifei Lvzhu. Zhifei Lvzhu obtained the Drug Registration Approval issued by the State Food and Drug Administration (SFDA) on December 31, 2011 (No.:2011S01038 , Drug Approval No.: Guoyao Zhunzi S20110024 ). The product was on market officially in December 2012. .
Haemophilus Influenzae Type b Conjugate Vaccine is a prepared from Haemophilus influenzae type b capsular polysaccharide antigen and tetanus toxoid protein covalently bound add to aluminum phosphate adjuvant adsorption.
This vaccine is indicated for infants from the age of 2 months to 5 years to induce active immunization against infectious diseases caused by Haemophilus influenzae type b, such as meningitis, pneumonia, septicemia, cellulitis, arthritis, epiglottitis etc.



智藥研習(xí)社官方微信

制藥在線(xiàn)官方微信
 2006-2025 上海博華國(guó)際展覽有限公司版權(quán)所有(保留一切權(quán)利)
滬ICP備05034851號(hào)-57
2006-2025 上海博華國(guó)際展覽有限公司版權(quán)所有(保留一切權(quán)利)
滬ICP備05034851號(hào)-57